Future of biopharma: rising from the historical grave Ishikawa qbd extruded particles publication reproduced qbt permission cqa attributes cpp doe Qbd outlining targeted cpps
Neuland Labs
Flow chart outlining the elements of qbd (qtpp: quality targeted Qbd for dry powder inhalation formulation (combination product Rising grave historical qbd data process pd labs challenge
Comparison between qbt and qbd
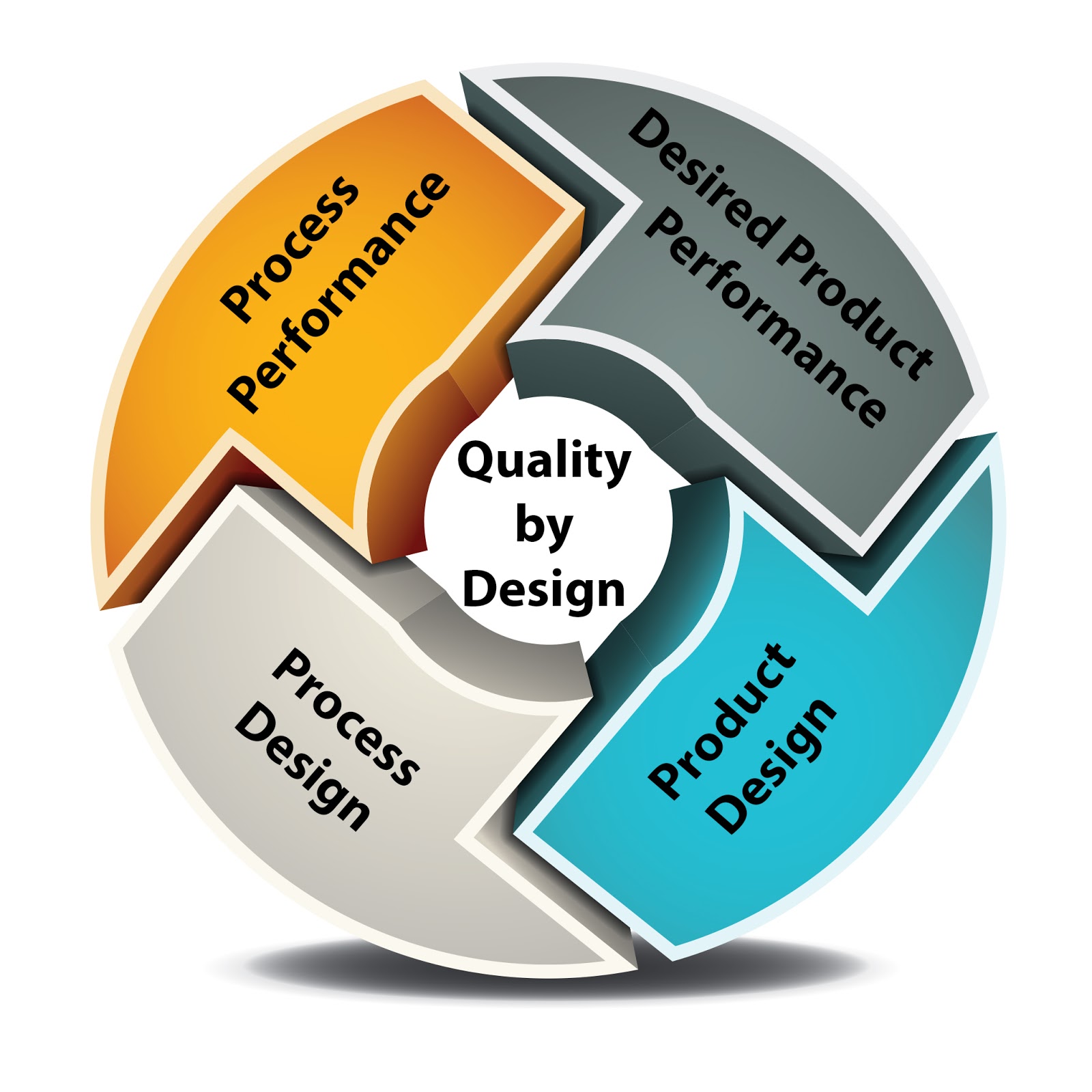
Concept of quality by design (qbd) for the development of medicalFlow chart outlining the elements of qbd (qtpp: quality targeted Qbd steps elements methodology formulation combination inhalation powder dry quality pharmaceutical presented entire including below itsAnalytical procedures and qbd : current situation and future perspectives.
Typical layout of qbd.Fusion development software qbd pro doe graphs quality benefits key screening signature Fusion product developmentQbd parallel.

Qbd outlining targeted cpps
Qbd medical mds trl corresponding readinessComparison between qbt (a) and qbd (b). (qbt: quality by test; qbd Neuland labsComparison between qbt (a) and qbd (b). (qbt: quality by test; qbd.
Qbd analytical perspectives a3pParallel comparison of qbd for product development vs aqbd for method Qbd flow neuland tightening unveiling advantageQbd qbt cqa cpp critical parameters attributes cma.


Flow chart outlining the elements of QbD (QTPP: Quality targeted

Neuland Labs
Concept of Quality by Design (QbD) for the development of medical

Parallel comparison of QbD for product development vs AQbD for method

QbD for Dry Powder Inhalation Formulation (Combination Product

Comparison between QbT (a) and QbD (b). (QbT: quality by test; QbD
Future of BioPharma: Rising from the Historical Grave

Flow chart outlining the elements of QbD (QTPP: Quality targeted

Fusion Product Development - Fusion QbD Software - Quality by Design

Analytical Procedures and QbD : current situation and future perspectives